AIM:1 :- To determine the absorption Maxima of paracetamol
Requirement
chemical paracetamol digital water.
Apparatus UV visible spectrometer volumetric flask Ambika glass road sector
Theory
the extent to which a simple absorbs light depends upon the wavelength of light the wavelength at which a substance show maximum absorption is called absorption maximum or ∆ lembda Max
The value of lambda Max is important for survival reasons
1.The wavelength is characterised of each compound.
2.it provides details about the electronic structure of the analyte
3.It insure highest sensor activity and minimises deviation from a low lambda Max can be determined by plotting graph between absorption and wavelength.
Procedure
Preparation of stock solution
(A)5 mg of paracetamol powder we are waiting using electronic balance
(B)This powder is then taken in a 50 ml volumetric flask and make up the mark by adding the sea water
(C)The flask must check that will until the present Amul powder has resolved the solution was stock solution
Preparation of work stock
Take 10 ml of stock solution and delete up 100 ml using distilled water
Method:-
- set the spectrometer wavelength to 20 NM and with a cuvette containing distilled water to set the instrument reference level
- Put the cuvette containing the prepared solution in the example compartment and record the absorbance
- Repeat step number to that wavelength in increments of 200 NM up to 400 nm and record the absorbance at each wavelength setting
- Cloud result and absorbance against wavelength
- From the graph note down the wavelength of maximum absorbance for the solution.
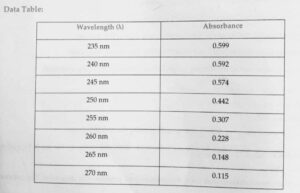
Result:-the absorption Maxima for the given sample solution was found to be …nm
